Minimalist pressure-controlled emergency ventilator for COVID-19
Severe COVID‑19 is frequently associated with hypoxemic acute respiratory failure. The number of patients needing invasive mechanical ventilation has surpassed in several countries the installed capacity and the existing manufacturers are having difficulties to address this increased demand putting at risk patient survival.
Additionally, the international procurement of the technical materials and components necessary to produce mechanical ventilators by other entities within the relevant timeframe and in the quantities required is likely to be difficult or impossible. It is then important that an emergency mechanical ventilator design emphasizes simplicity and local availability of components and manufacture, while simultaneously providing an effective and safe ventilatory support.
On the other hand, medical mechanical ventilators are normally sophisticated machines for general use, although this level of sophistication is not needed to save lives. Indeed, several governments have issued guidelines for simplified mechanical ventilators for COVID-19.
Our team of medical doctors and engineers identified a ventilation mode that is at the same time effective and safe for COVID-19 emergency clinical intensive care and technically easy to implement, in face of the practical constraints mentioned above. Such mode is the pressure‑controlled continuous mandatory ventilation mode (PC-CMV) with pure oxygen.
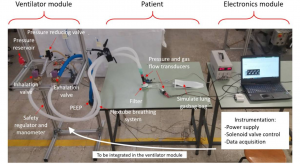
Our proof-of-concept devices use only two types of technical components: a gas regulator and two identical electrovalves, capable to deliver PC-CMV in a double circuit system. The rest of the device can be produced with only modest technical means, involving simple mechanics and electronics.
The quantitative tests performed have shown that the pressure curves thus achieved are acceptable for the purpose in view.
The technical components used are meant either for gas distribution in buildings or for low pressure fluids such as water, and are ubiquitous. Other types of regulators and valves, possibly more suitable for the purpose, can also be used if available.
It should be clear that the components involved, although being sturdy industrial devices, are most likely not medical-grade or certified for use in oxygen, so this proposal can only be understood as a last resort solution for a temporary emergency.
Besides the procurement of materials, manufacture and components, some engineering still needs to be done on the pathway to a practical ventilator: physical- and bio-compatibility of the materials, durability, sterilizability, safety, fail-handling and general ergonomics. In view of the urgency, these developments should be restricted to the barest minimum for the end in sight.
https://arxiv.org/abs/2004.00310
Keywords
intrusive ventilator, emergency clinical intensive care,
Associated Users
Location
Coimbra, Lisbon
Entities
Laboratório de Instrumentação e Física Experimental de Partículas (LIP – Coimbra) Faculdade de Ciências e Tecnologia da Universidade NOVA de Lisboa (FCT NOVA) Nova Medical School (NMS) Haas F1 Team Project OpenAir
0 Comments